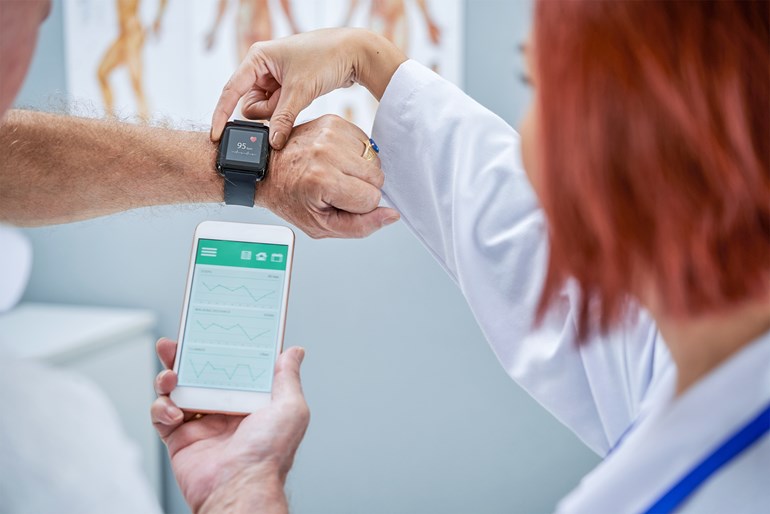
In recent years, the level of innovation and growth in wearable technologies has led increasingly to the use of this technology in healthcare and clinical trial settings (1). Additionally, the application of health monitoring devices in clinical research is proving an important element in allowing some trials to continue during this time of global pandemic (2).
Initially conceived with consumers in mind, wearable technology devices have created a world of opportunity for the pharmaceutical industry. With the relaxation of FDA regulations concerning monitoring requirements made in response to the COVID-19 pandemic (3), wearable technology may give clinical researchers greater flexibility in meeting their regulatory and reporting requirements while improving the trial process for participants.
For many years, projections for the global wearable healthcare market have suggested significant growth. Forecasts from mid-2019 indicated the market will be worth $139.4bn in 2026, having grown from $24.6bn in 2018 (4). Clinical research is now experiencing a paradigm shift as this consumer-driven increase in mobile health (mHealth) provides a means of continuing operations within budgetary and logistical constraints. As clinical research becomes more decentralized and trial managers interact less with participants, technologies such as wearables and remote sample collection are be-coming increasingly valuable to trial completion.
In this article, we discuss the benefits and challenges regarding the application of wearable devices in clinical research and the value of embracing a decentralized approach to trials.
Consumer healthcare wearables
Consumer healthcare wearables include smart phone apps, chest straps, sports watches, patches, and other monitoring sensors that can be worn on the body such as pulse oximeters (5). These products track users’ physical activity using smart and/or GPS technology and measure essential health data such as heart rate, respiratory rate, blood oxygen saturation level and body posture. In one re-cent clinical example, Pfizer was able to send eczema patients home with wearable trackers to measure how often they were scratching in their sleep, rather than monitoring them at sleep clinics (6).
Other technologies include Apple’s ResearchKit, which provides an open-source software framework that allows researchers to create apps for clinical data collection via wearable devices and smartphones (7).
The integration of wearable health monitors with smartphones offers increasing capabilities to gather and store health data remotely and in real time. As long as a person is operating and/or wearing the device, data can be collected continuously to provide larger samples without the need for in-clinic visits. This remote monitoring increases the speed and efficiency of data collection, and facilitates off-site monitoring of trial participants.
When all of the wearable tech results are combined, these samples also give a fuller picture of a per-son’s health and performance than would a single point of data, potentially providing more accurate information to researchers within a shorter time frame. Additionally, they may improve patient recruitment and retention for trials by minimizing the requirements on patients to manually collect data or to make clinical site visits.
Increased use of wearables in clinical trials
An increasing number of pharmaceutical and biotechnology sponsors are already using wearables to realize the potential benefits (8). As of 2019, around 1,400 clinical trials had been conducted using wearable devices (9) and that number is likely rising as the healthcare industry increasingly conducts trials virtually, and gathers data remotely, in response to COVID-19.
With remote monitoring, good management of clinical trial logistics becomes essential to trial success. Clinical services providers like Avantor will increasingly need to deliver through multiple supply streams to help researchers improve efficiency and productivity throughout the trial life-cycle. If the logistical considerations are managed correctly through a partner capable of meeting these challenges, then current and future clinical research will be well-placed to benefit from these new technological innovations.
Studies using wearables involve therapeutic areas including asthma, cancer, schizophrenia, and diabetes. A couple of examples include:
• Quality-of-Life Tracking
In 2018, a team at the Cedars-Sinai Samuel Oschin Comprehensive Cancer Institute used wrist-mounted trackers to monitor a group of advanced cancer patients over six months. Results from the study suggested that fitness trackers can help assess quality of life for patients and provide more accurate and ongoing medical monitoring. The devices also reduced the risk of bias and could potentially enable earlier intervention when necessary and improve treatment outcomes. The team are now planning to study long-term use of these devices in a larger and more diverse patient group (10).
• Virtual Trial for Heart Medication
Janssen announced in late 2019 that they would be conducting their first-ever completely virtual clinical trial. The CHIEF-HF trial, to assess the use of Invokana (canagliflozin) in adults with heart failure, will use smart technology and wearable devices to gather and analyse data, and participants will engage with the study virtually on their smartphones (11).
Challenges of mHealth in clinical trials
As more clinical researchers embrace wearable devices, the increased adoption of mHealth in trials emphasizes the challenges to be considered (12):
• Security and privacy − what are the risks of private patient data being accessed by the public?
• Data accuracy and validation of data − has the patient worn the device at all relevant times during the trial without tampering or hindrance?
• Ethical issues, such as gaining approval and ownership over the information generated.
• Supply of the devices, including delivery and maintenance, can be impacted by unexpected constraints on the supply chain – requiring trials to work with suppliers with the capacity to adjust to rapidly changing circumstances.
• The logistical considerations of effective implementation of mHealth technology on a global scale.
While clinical researchers need to consider the implications of these challenges on upcoming trials, proper supply chain management can do a great deal to mitigate the undesirable consequences of remote trial research. Clinical trial managers can maximize the likelihood of trial success by enlisting a clinical service provider with expertise in product sourcing, trial management, and logistics supply, and who can deliver the correct trial equipment on time and in full. This will enable trials to take ad-vantage of the considerable benefits of remote monitoring for both trial participants and researchers.
Benefits for clinical researchers
Despite the challenges of mHealth, when used in single blinded trials, biometric data collected from medical healthcare wearable devices can offer researchers several qualitative and practical benefits including:
• Accurate remote monitoring of participants with data being transmitted digitally, eliminating the need for clinical site visits and making it easier for trials to continue despite unforeseen global or local crises.
• A real-world view of a patient’s response to treatment with ongoing monitoring of health status and response to the trial drug that is more objective than the patient recording their experience anecdotally.
• Earlier decision-making by providing access to near continuous real-time data. For example, researchers may make amendments to protocols based on how participants react to a drug in real time.
• More accurate intervention triggers by alerting researchers to potential adverse events or patient non-compliance sooner during the trial.
• An improvement in subject retention by delivering prompts and sharing information to en-courage active patient participation.
• Reduced costs by decreasing the time and expense of clinic visits, as well as accurate and in-depth participant monitoring despite decentralized trial management.
• Data from mobile sensors can provide researchers with extra information to show the benefits of a drug, opening up the potential for different approaches to research.
• Utilizing wearables and other at-home monitoring supplies, all relevant data points can be collected securely and remotely, allowing trials to continue smoothly regardless of circumstance.
Improving the experience for patients
Wearable medical devices allow data to be collected digitally and automatically so patients may not need to keep manual data records. They may also be under less pressure to recall this information at a later date.
Significantly, using wearable health devices can also help reduce the need for clinical visits, giving participants a greater degree of independence. This increases the likelihood that patients will, or can, enroll in a trial, and help the trial complete on time regardless of limits to the collection of data.
Pharmaceutical companies and Clinical Research Organizations are not alone in facing the challenges of conducting decentralized clinical trials. As researchers embrace remote monitoring, clinical supply chain partners can help trials succeed by providing the reliable procurement and distribution of quality clinical wearables and other remote monitoring supplies.
As the COVID 19 outbreak has shown, supply chains and manufacturing processes are vital to limiting the impact of logistical restrictions and supply reductions on clinical trials. Clinical suppliers who can properly manage these potential risks – and deliver all required equipment on time, even in at-home settings – play an important role in freeing researchers up to focus on patient outcomes. From the supply of clinical wearables and at-home sample collection kits, to the efficient supply and management of equipment – and ultimately the secure storage of samples and data in a biorepository and archiving facility – the future of clinical research will rely on the ability of trials and suppliers to adapt to a more flexible approach to achieving trial objectives.
Summary
The landscape in which clinical trials are conducted is constantly evolving, becoming more decentralized, and wearable healthcare devices offer valuable data collection to supplement more traditional methods of research. They provide accurate remote results, allowing researchers to personalize treatment for specific patient groups.
In addition, the use of wearable medical devices and remote sample collection in clinical trials offers participants a safer and more convenient experience, and can encourage patient enrollment and participation in future trials. Trial service providers who understand the evolving needs of the clinical landscape, such as Avantor Clinical Services, will be invaluable partners in helping researchers see trials through to in successful outcomes.
This article has been produced by Avantor Clinical Services as part of our ongoing commitment to setting science in motion to create a better world through the delivery of mission-critical products and services. To learn more, contact us today.
References
1. Niklas Morton, David Blackman. The Growing Availability of Wearable Devices: A Perspective on Current Applications in Clinical Trials. May 2016. http://www.appliedclinicaltrialsonline.com/growing-availability-wearable-devices-perspective-current-applications-clinical-trials. Accessed Apr 2020.
2. Enforcement Policy for Non-Invasive Remote Monitoring Devices Used to Support Patient Monitoring During the Coronavirus Disease-2019 (COVID-19) Public Health Emergency. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-non-invasive-remote-monitoring-devices-used-support-patient-monitoring-during. Ac-cessed May 2020.
3. Ibid.
4. Fortune Business Insights Wearable Medical Devices Market. https://www.fortunebusinessinsights.com/industry-reports/wearable-medical-devices-market-101070. Accessed Apr 2020.
5. Elena S. Izmailova, John A. Wagner, Eric D. Perakslis. Wearable Devices in Clinical Trials: Hype and Hypothesis. July 2018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032822/. Ac-cessed July 2020.
6. Sandeep Menon. How Pfizer Is Using Wearables To Understand The Patient Experience. March 2020. https://www.clinicalleader.com/doc/how-pfizer-is-using-wearables-to-understand-the-patient-experience-0001. Accessed May 2020.
7. ResearchKit and CareKit. Apple. https://www.apple.com/researchkit/. Accessed July 2020; Ed Miseta. GSK Uses Apple ResearchKit in Rheumatoid Study. https://www.clinicalleader.com/doc/gsk-uses-apple-researchkit-in-rheumatoid-study-0001. Accessed Apr 2020.
8. Ryan Kraudel. Wearables in Clinical Trials: Opportunities and Challenges. April2019. https://valencell.com/blog/2019/04/wearables-in-clinical-trials-opportunities-and-challenges/. Accessed July 2020.
9. Ibid.
10. Fitness trackers prove helpful in monitoring cancer patients. https://www.sciencedaily.com/releases/2018/07/180724174304.htm. Accessed April 2020.
11. Anna Smith. Janssen pilots wearable technology in clinical trial. November 2019. http://www.pharmatimes.com/news/janssen_pilots_wearable_technology_in_clinical_trial_1317368. Accessed May 2020.
12. Food and Drug Administration. Intent To Exempt Certain Unclassified, Class II, and Class I Re-served Medical Devices From Premarket Notification Requirements. August 2015. https://www.federalregister.gov/documents/2015/08/14/2015-20005/intent-to-exempt-certain-unclassified-class-ii-and-class-i-reserved-medical-devices-from-premarket. Accessed Apr 2020.
potential of wearable technology for the future of clinical research